Details of the Drug
General Information of Drug (ID: DMAPO0T)
| Drug Name |
Ciglitazone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ciglitazone; 74772-77-3; Ciglitizone; ADD-3878; Ciglitazonum; Ciglitazona; Ciglitazonum [Latin]; Ciglitazona [Spanish]; Ciglitazone [USAN:INN]; ADD 3878; CHEBI:64227; U-63287; (+-)-5-(p-((1-Methylcyclohexyl)methoxy)benzyl)-2,4-thiazolidinedione; C18H23NO3S; 5-(4-((1-methylcyclohexyl)methoxy)benzyl)thiazolidine-2,4-dione; YZFWTZACSRHJQD-UHFFFAOYSA-N; (+/-)-5-[4-(1-Methylcyclohexylmethoxy)benzyl]thiazolidine-2,4-dione; U 63287; 5-{4-[(1-methylcyclohexyl)methoxy]benzyl}-1,3-thiazolidine-2,4-dione; NCGC00164446-01
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
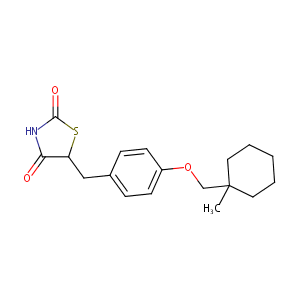 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 333.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pulmonary fibrosis | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CB03.4 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
